Literature Review of the Solid Oxide Fuel Cell
Computational Study of Protien-ligand Interactions in Cancer: Literature Review
August 10, 2021Literature Review on Single Pilot Operations
August 10, 2021Literature Review of the Solid Oxide Fuel Cell
Literature review outline
- Solid Oxide Fuel Cell: basic principles; most used materials for anode, cathode and electrolyte
- SOFC systems including a Thermal Energy Storage
- PCM overview and PCMs for high temperature applications
- Thermal Energy Storage based on PCMs
1. Solid Oxide Fuel Cell
1.1 Overview
Solid oxide fuel cells (SOFCs) are the most efficient devices yet invented for conversion of chemical fuels directly into electrical power [1]. They consist of a solid dense ceramic electrolyte placed between two porous electrodes. The fuel is supplied to the anode side, air or oxygen to the cathode. The absence of a liquid electrolyte allows to eliminate one of the source of electrode corrosion and in general all the issues linked to the management of a liquid electrolyte (evaporation, for instance). Furthermore, simpler fabrication techniques can be adopted if the cell is a completely solid-state device. As concerns cell geometry, there are two main designs (Figure 1 and Figure 2) and each has its advantages.
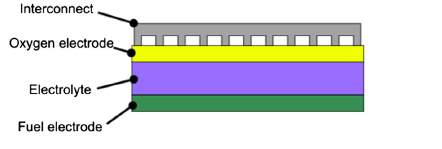
Figure 1. Planar configuration
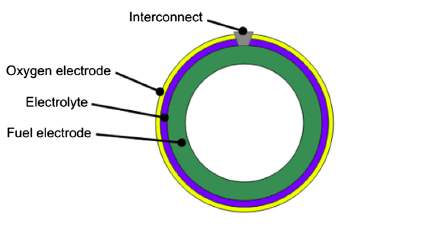
Figure 2. Tubular configuration
The planar cell is more widespread thanks to the higher volumetric power density, shorter electrons paths and cheaper cost [2]. The tubular design, instead, presents a higher thermomechanical stability. Planar design, in turn, can be of two types: electrolyte and electrode supported. In the electrolyte supported cell, the mechanical strength is ensured by the electrolyte. For this reason it is thicker (100-200 μm) than the other cell components. The working temperatures can reach 1000 °C in order to further increase the electrolyte ionic conductivity and so counterbalance the higher ohmic losses. As regards the electrode supported cell, cathode or anode are the thickest component of the cell (200-1000 μm). Therefore, due to a thinner electrolyte, the operating temperatures can be lower (700-800 °C) and the durability of the cell improves.
Each component of the cell must exhibit certain characteristics for the system to function effectively [3]. In the following lines, the requirements of each component are presented:
- electrolyte: it has to be characterised by high ionic conductivity and, at the same time, low electronic conduction at operating temperatures in order to reach good current efficiencies [4]; it must be sufficiently dense to be impermeable to gases, so avoiding H2 and O2 recombination; it should be chemically and mechanically stable at operating conditions; its thermal expansion coefficient has to be comparable to those of electrodes.
- electrodes: high electronic conductivity at operating temperatures; considerable porosity and adequate pores size to ensure an enough number of reaction sites (TPB) and simultaneously to promote products removal; optimised chemical composition and morphology to allow efficient transport of electrons and ions. In more detail: cathode composition must be such as to catalyse oxygen reduction and guarantee chemical stability in reducing condition; anode microstructure instead has to promote fuel oxidation and warrant chemical stability in oxidizing environment.
When an oxygen molecule comes into contact with the cathode/electrolyte interface, it reacts with the free electrons at the cathode, forming O2- ions. These oxide ions, which represent the charge carriers, are then transferred from the cathode through the electrolyte to the anode [5]. Here, they interact with hydrogen to produce water, releasing electrons to an external circuit. The half-cell reactions occurring at anode and cathode, and the total reaction in an SOFC operated with hydrogen, are:

